
ĭmitri Mendeleev, probably unaware of earlier work on a periodicity of the elements, arranged in the early 1860s a table that contained triads similar to those of Döbereiner. In 1864 this was followed by John Newlands’ law of Octaves.

Several metals of the PTE, that is, their ions and complexes, are employed in medicine and we discuss the role of lithium, gallium, strontium, technetium, silver, gadolinium (the only f-block element), platinum, and gold.Īnd noted that the members in a group, which was arranged according to the increasing atomic weights, had related properties. All metals of the PTE and a plethora of their compounds are used in industry and many of them are highly toxic, like lead (Pb), which is discussed as a prime example. Two other metalloids, silicon and arsenic, are briefly mentioned, but they have not been proven as being essential for humans. From the p-block metals only the metalloid (half-metal) selenium (Se) is essential for all forms of life.
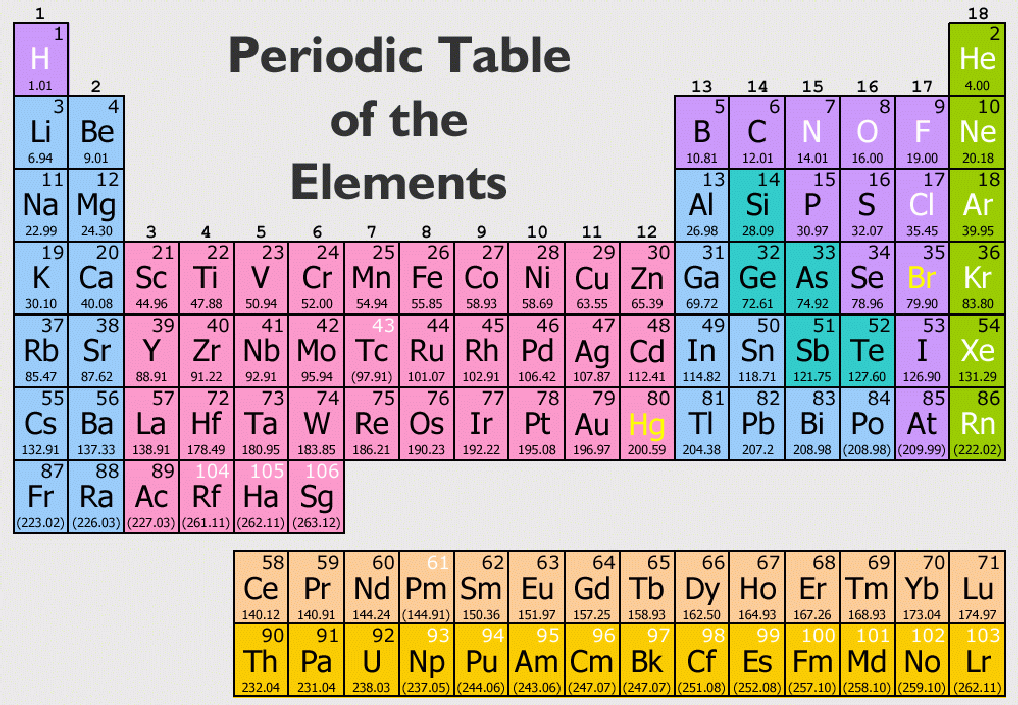
Chromium is no longer classified as being essential. The trace elements of the d-block of the PTE as far as they are essential for humans (Mn, Fe, Co, Cu, Zn, Mo) are emphasized, but V, Ni, Cd, and W, which are essential only for some forms of life, are also considered. The bulk elements sodium (Na), potassium (K), magnesium (Mg), and calcium (Ca) from the s-block, which are essential for all kingdoms of life, and some of their bio-activities are discussed. The bio-relevant metals (and derived compounds) of the Periodic Table of the Elements ( PTE) are in the focus.


 0 kommentar(er)
0 kommentar(er)
